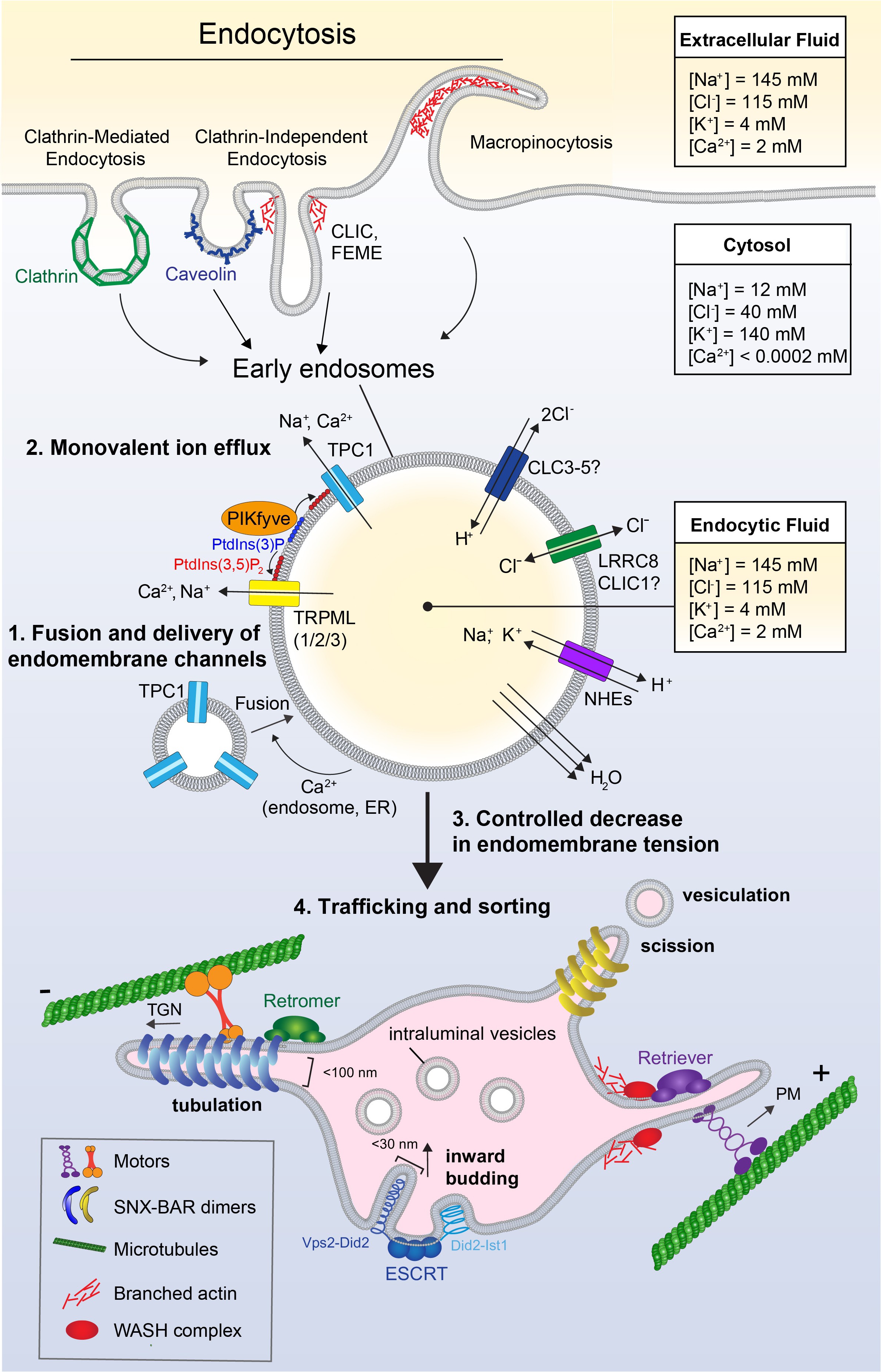
Fig. 2. Endocytosis, ion flux, and membrane dynamics in early endosomes. Extracellular fluid can be internalized through a variety of mechanisms such as clathrin-mediated endocytosis, clathrin-independent endocytosis, or macropinocytosis, forming the nascent early endosome. As the endosome matures, it undergoes Ca2+-mediated homotypic fusion with other (early) endosomes and vesicles containing additional cargo and endomembrane channels (1). Compared to the cytosol, the concentrations of ions such as Na+, Cl-, and Ca2+ are relatively high in the extracellular fluid and the resultant endosome, which are round and minimize surface:volume. As membrane is recycled back to the surface, these ions are removed from endosomes to decrease their volume and prevent an osmotic pressure that causes membrane tension (2). Monovalent ion flux occurs through the action of lipid-gated cation channels such as TRPML1/2/3 and TPC1, ion co-transporters such as ClCs and NHEs, and potentially other anion channels such as VRAC/CLIC. Lipid-gated cation efflux further relies on the action of PIKfyve, which phosphorylates PtdIns(3)P to form PtdIns(3,5)P2, to then gate the conductance of cation channels TRPML and TPC. As ions are removed from the early endosome, this leads to osmotically-driven water loss and shrinkage of the compartment. Volume loss decreases endomembrane tension, creating slack in the membrane and allowing its remodeling through tubulation, vesiculation, and budding (3). Endocytic cargo is then sorted; Retromer and Retriever complexes recognize and sort cargo destined for recycling and segregate this cargo into subdomains which undergo tubulation (4). These tubules have a high surface:volume ratio, allowing the effective concentration of membrane-bound cell surface receptors to recycle back to the plasma membrane. The forces needed for tubulation are generated by concerted actions of the actin cytoskeleton, including actin polymerization by WASH, and pulling forces of motor proteins moving along microtubules. Endomembrane tubules are coated and stabilized by curvature-sensing proteins containing with BAR domains These tubules undergo scission and vesiculation, leaving behind cargo destined for degradation. Remaining cargo can be recognized by ESCRT complexes, which also utilize membrane slack in order to deform the membrane and generate inwardly-budding intraluminal vesicles (ILVs). Inward budding is supported by corkscrew-shaped protein complexes which polymerize and pull the membrane into narrow tubules that eventually undergo scission to form ILVs. These ILVs form the basis of the multivesicular late endosome, which later fuses with the lysosome for degradation.